Looking for the latest ENFit update? Click here.
The enteral device industry is undergoing a major change in 2015/2016. You will notice differences in the feeding sets, syringes and feeding tubes that you use over this coming year and next. These changes are referred to as ENFit.
Shield HealthCare wants to make sure that you are aware of these changes. Read on to find out more!
WHAT is ENFit?
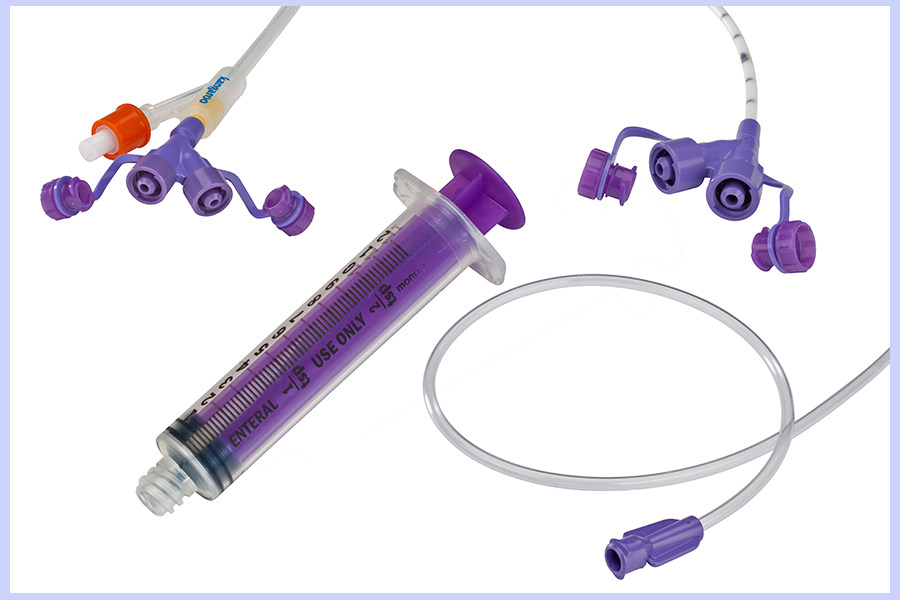
To improve patient safety, manufacturers of enteral devices are required to change the way enteral feeding sets and syringes connect to feeding tubes. The name of the new connection is ENFit.
Enteral tubing misconnection occurs when enteral devices (feeding bags, tubes or syringes) are connected to non-enteral devices, such as IV lines, urinary catheters and ventilator tubing.
ENFit is the new connection standard, developed by an international group of clinicians, manufacturers and regulators as part of the Stay Connected initiative to prevent tubing misconnections.
WHY change?
The Joint Commission identifies tubing misconnection as a sentinel (high risk) event.
ENFit connectors will not allow accidental connection between enteral and non-enteral devices.
WHEN will this happen?
The transition from current enteral devices to ENFit will occur over three phases in 2015 and 2016.*
Each phase will include a change to a different part of the enteral feeding system.
New ENFit Enteral Connector Timeline:A
And the new schedule is as follows:
First Quarter (January – March) 2016
- Enteral-specific syringes with ENFit tip
- Enteral Feeding tubes with ENFit connectors
Transitional stepped connectors and current syringes will remain available while patients make the transition to the new feeding tubes.
Please consult your supplier for product-specific availability and indications.
*All dates are projected estimates and subject to change due to timing of product-specific regulatory review and manufacturer discretion.
Here’s how Shield HealthCare is helping to increase awareness in the community about these changes:
-
- Training for healthcare professionals
-
- Training for Shield HealthCare staff and representatives
-
- Notification letters to customers affected by these changes
- Close communication with manufacturers
Click here for a full-size printable PDF of the postcard below: Postcard (please be advised that the dates have changed since the creation of this PDF).
For more information, visit Stay Connected for frequently asked questions and the latest updates on this initiative.
Find more information from Shield HealthCare about ENFit and tube feeding here:
- Are You Ready for ENFit? New Feeding Tube Connectors: Video
- Preventing Enteral Misconnections with ENFit® Connectors
- Tube Feeding A Child: The G-Tube That Saved My Son’s Life & My Sanity
- Recorded Webinar: The Complete Nurse’s Guide to Tube Feeding
- Tube Feeding at School: 8 Tips to Prepare Your Child and School Staff























Seems more and more medical companies that stand to make a profit selling commercial formulas are jumping on the ENFit connector band wagon. Some food for thought before you place too much emphasis on ENFit being the safety solution for small bore feeding tube connectors.
1. ENFit connectors will create another safety issue once implemented that is larger than the less than 10% misconnection issue. It’s called tube pull outs, and is a result of having the feeding tube snag on any obstruction, (I.e IV pole, door knob, chair rail, bed post, car door jam, brushing up against another person while in a crowed elevator, or walkway). Currently with the existing connector if a snag happens the feeding tube disconnects saving a feeding tube pullout. Once the ENFit connector becomes an everyday connector and is twist locked so it will not disconnect then pull outs will be happening more and more. Causing unnecessary exchanges, stoma and stoma channel damage. ER visits and RIG or PEG procedures to place new tubes. There is more safety issues if you are interested check out ENFit issues on the web.
The supplier of my enteral supplies, Lincare in Jacksonville, Florida did not inform me of upcoming changes. So, I received a supply of bags with the new connector. Finally learned after a couple of weeks about the change and had to rely on internet for informaton. This interim connector doesn’t stay connected. HATE IT!
I am afraid of the Enfit connector because it will only cause more tube pull-outs. Wishing we users had a say before these changes were made. Had no problem with existing connectors, bags, syringes.
Will multiple companies be providing the supplies or does one company have a monopoly…that’s what its looking like.
The new connections seem rather small in diameter of the passage. This could be a serious problem for people like me who puree real food rather than use the corn syrup poison that is traditional PEG formula. The traditional formula destroyed my pancreas in 18 months due to sugar being the only source of calories in the formula. I started blending my own food and using a fresh food formula called Liquid Hope. Since then my glucose and A1C has been manageable (though I am still fighting it – I was never diabetic before as I was an athlete and always ate healthy). This new connection seems to solve a relatively nonexistent problem, but rather to force more people to use the traditional corn syrup formulas.
Who will sell nutrisafe adapter so kangaroo feeding bags are capatible with gj feeding tube ports. The new enfit will not fit in the ports.